TRODELVY® (sacituzumab govitecan-hziy) is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract that have spread (metastatic) or cannot be removed by surgery, and who have received a platinum-containing chemotherapy medicine and also received an immunotherapy medicine. This indication is approved based on medical studies that measured how many patients responded and how long they responded. Continued approval may depend on benefit demonstrated in additional medical studies. It is not known if TRODELVY is safe and effective in people with moderate or severe liver problems or in children.
TRODELVY is designed to work differently than traditional chemo
TRODELVY targets cells with Trop-2
TRODELVY is a type of treatment called an antibody-drug conjugate (ADC). An ADC is a substance that binds to a specific protein or receptor found on certain types of cells, including cancer cells. Scientists discovered that tumor cells in certain cancers have a higher amount of proteins called Trop-2 than normal cells (or noncancer cells). TRODELVY is designed to bind to cells with Trop-2 and deliver powerful anticancer medicine.
Information from laboratory studies suggests that this is how TRODELVY works. The clinical benefit of these observations is unknown.
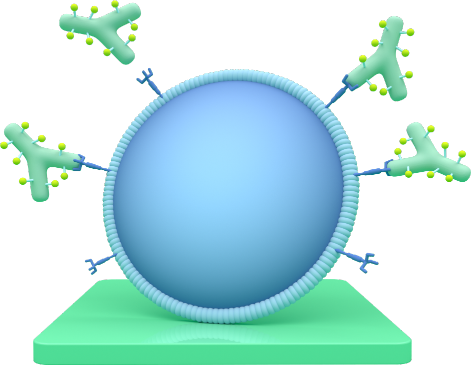
Seeks out
TRODELVY attaches to Trop-2.
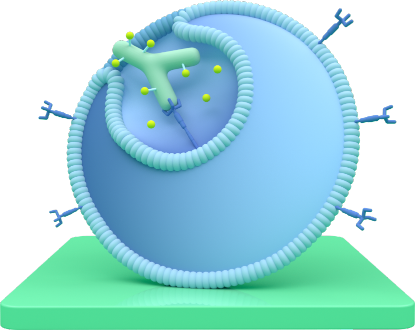
Breaks in
Once attached, TRODELVY enters the cancer cell.
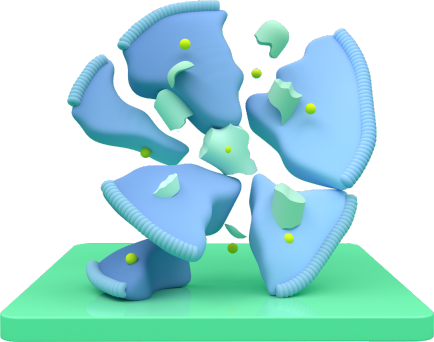
Destroys
Once TRODELVY enters, the anticancer medicine is released, killing the cell.
How TRODELVY works
Understanding what makes each treatment different can be confusing. This video will help break down the science to show you how TRODELVY is designed to work. Think of TRODELVY like a cargo ship designed to deliver anticancer medicine directly into cells with Trop-2 proteins. Watch to learn about TRODELVY from an oncologist and some unexpected guests.
<TEXT ON-SCREEN>
HOW TRODELVY IS DESIGNED TO WORK
TRODELVY®
sacituzumab govitecan-hziy
180 mg for injection
WHAT IS TRODELVY?
TRODELVY® (sacituzumab govitecan-hziy) is a prescription medicine used to treat adults with:
- triple-negative breast cancer (negative for estrogen and progesterone hormone receptors and HER2) that has spread to other parts of the body (metastatic) or cannot be removed by surgery, and who have received two or more prior treatments, including at least one treatment for metastatic disease.
- hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative breast cancer that has spread to other parts of the body or cannot be removed by surgery, and who previously received endocrine therapy and at least two additional treatments for metastatic disease.
- bladder cancer and cancers of the urinary tract that have spread or cannot be removed by surgery, and who have received a platinum-containing chemotherapy medicine and also received an immunotherapy medicine. This indication is approved based on medical studies that measured how many patients responded and how long they responded. Continued approval may depend on benefit demonstrated in additional medical studies.
It is not known if TRODELVY is safe and effective in people with moderate or severe liver problems or in children.
IMPORTANT SAFETY INFORMATION
TRODELVY can cause serious side effects, including low white blood cell count and diarrhea:
- Low white blood cell count (neutropenia) which is common and can sometimes be severe and lead to infections that can be life-threatening or cause death. Your healthcare provider should check your blood cell counts during treatment. If your white blood cell count is too low, your healthcare provider may need to lower your dose, give you a medicine to help prevent low blood cell count with future doses of TRODELVY, or in some cases may stop TRODELVY. Your healthcare provider may need to give you antibiotic medicines if you develop fever while your white blood cell count is low. Call your healthcare provider right away if you develop any of the following signs of infection: fever, chills, cough, shortness of breath, or burning or pain when you urinate.
- Severe diarrhea. Diarrhea is common and can be severe. Severe diarrhea can lead to loss of too much body fluid (dehydration) and kidney problems. Your healthcare provider should monitor you for diarrhea and give you medicine as needed to help control it. If you lose too much body fluid, your healthcare provider may need to give you fluids and electrolytes to replace body salts. If you develop diarrhea during your treatment with TRODELVY, your healthcare provider should check to see if it may be caused by an infection. Your healthcare provider may decrease your dose or stop TRODELVY if your diarrhea is severe and cannot be controlled with anti-diarrheal medicines.
- Call your healthcare provider right away the first time that you get diarrhea during treatment with TRODELVY; if you have black or bloody stools; if you have symptoms of dehydration, such as lightheadedness, dizziness, or faintness; if you are unable to take fluids by mouth due to nausea or vomiting; or if you are not able to get your diarrhea under control within 24 hours.
Do not receive TRODELVY if you have had a severe allergic reaction to TRODELVY. Ask your healthcare provider if you are not sure.
Please continue watching for Important Safety Information at the end of this video and please see link provided for Important Facts, including Important Warning.
The actors portrayed in this video are not actual TRODELVY patients. They are here for your education and entertainment. Enjoy.
<END OF TEXT ON-SCREEN>
Crystal:
I’m an info nerd. UFO sightings, beekeeping, or Barry Manilow’s greatest hits, you name it, I know it. So when I got my metastatic cancer diagnosis, I wanted to know everything I could about treatment options.
<TEXT ON-SCREEN>
TRODELVY®
sacituzumab govitecan-hziy
180 mg for injection
<END OF TEXT ON-SCREEN>
Right now, I’m looking at some firsthand experience with TRODELVY sacituzumab govitecan-hziy?
<TEXT ON-SCREEN>
sacituzumab govitecan-hziy
<END OF TEXT ON-SCREEN>
Is that a word? Has anyone here tried it?
<TEXT ON-SCREEN>
CRYSTAL
Curious about TRODELVY
Self-proclaimed Info Nerd
<END OF TEXT ON-SCREEN>
<TEXT ON-SCREEN>
TRODELVY? I haven’t tried that yet.
I’ll have to talk to my doctor about that one.
My doctor mentioned TRODELVY, but I haven’t looked into it.
I’ve been taking TRODELVY for three months now. What’s on your mind?
I’ve heard about TRODELVY but need to find more info too. Let me know what you learn.
<END OF TEXT ON-SCREEN>
Amanda:
I’ve been taking TRODELVY for 3 months now. What’s on your mind?
<TEXT ON-SCREEN>
AMANDA
TRODELVY Patient and Champion
<END OF TEXT ON-SCREEN>
Crystal:
I’ve probably got 100 questions, but how exactly does it work?
Amanda:
My doctor told me it delivers anticancer medicine into cells... But how it does that… I’m lousy at explaining things. Hold on one moment.
<TEXT ON-SCREEN>
My doctor told me it delivers anticancer medicine into cells…
<END OF TEXT ON-SCREEN>
<TEXT ON-SCREEN>
DR. ESPOSITO
Oncologist
(Has no idea she’s on mute)
<END OF TEXT ON-SCREEN>
Amanda:
You’re on mute.
Dr. Esposito:
Sorry. Anyway, hey. I’m an oncologist. It is so good to see you again. Do more people want to learn about TRODELVY? That’s 4 just this week!
Crystal:
Wait, did you two plan this?
Dr. Esposito:
First, let’s look at cancer cells. They’re notoriously hard to destroy, smart, and may become resistant or may not respond at all to treatments.
TRODELVY takes a different approach when it comes to cancer cells.
Crystal:
Okay. I’m with you so far.
Dr. Esposito:
TRODELVY is known as an antibody-drug conjugate or ADC for short.
<TEXT ON-SCREEN>
Antibody-drug conjugate
ADC
<END TEXT ON-SCREEN>
Amanda:
Uh, anti-what now?
Dr. Esposito:
Think of TRODELVY like a cargo ship. The TRODELVY ship is an antibody, and it’s carrying anticancer medicine.
Jim:
Woah, woah—not to knock the wind out of your sails, but I know a thing or two about ships. They need some type of navigation to see where they’re going. See, there was this one time I was lost at sea, and I had to navigate by sense of smell. We had nothing to eat but sea sponges and…
<TEXT ON-SCREEN>
JIM
TRODELVY patient
Lost at sea
<END TEXT ON-SCREEN>
Dr. Esposito:
I can’t speak to the nutritional benefit of a sea sponge-based diet. Our SS TRODELVY is an antibody designed to seek out a specific protein called Trop-2. Compared with healthy cells, some cancer cells may have a higher amount of Trop-2.
Jim:
Trop-2... Do I need to see the first Trop to understand the sequel?
Amanda:
Good one. So these Trop-2 things enable TRODELVY to target cells?
Dr. Esposito:
The Trop-2 acts like a beacon, telling the TRODELVY ship to enter the docking area of that specific cell.
Jim:
What kind of cargo is this TRODELVY ship carrying anyway?
Dr. Esposito:
The cancer-fighting kind. Each TRODELVY ship can on average carry about 7 to 8 molecules of the anticancer medicine.
Crystal:
Holy ship!
Dr. Esposito:
Now, once TRODELVY docks in the cell, it delivers and releases the anticancer medicine, destroying the cell.
Crystal:
Wow! So TRODELVY is targeted at those cells with Trop-2? That sounds like a treatment option I’d like to try. I’ll discuss it with my doctor.
Dr. Esposito:
Information from laboratory studies suggest that this is how TRODELVY works. The clinical benefit of these observations is unknown.
<TEXT ON-SCREEN>
Information from laboratory studies suggest that this is how TRODELVY works. The clinical benefit of these observations is unknown.
<END OF TEXT ON-SCREEN>
Crystal:
Thanks for talking me through how TRODELVY is designed to work. Is there any important safety information I should know about?
[Voiceover with text on-screen]:
I’m glad you asked. Here’s what you should know.
IMPORTANT SAFETY INFORMATION
TRODELVY can cause serious side effects, including low white blood cell count and diarrhea:
- Low white blood cell count (neutropenia) which is common and can sometimes be severe and lead to infections that can be life-threatening or cause death. Your healthcare provider should check your blood cell counts during treatment. If your white blood cell count is too low, your healthcare provider may need to lower your dose, give you a medicine to help prevent low blood cell count with future doses of TRODELVY, or in some cases may stop TRODELVY. Your healthcare provider may need to give you antibiotic medicines if you develop fever while your white blood cell count is low. Call your healthcare provider right away if you develop any of the following signs of infection: fever, chills, cough, shortness of breath, or burning or pain when you urinate.
- Severe diarrhea. Diarrhea is common and can be severe. Severe diarrhea can lead to loss of too much body fluid (dehydration) and kidney problems. Your healthcare provider should monitor you for diarrhea and give you medicine as needed to help control it. If you lose too much body fluid, your healthcare provider may need to give you fluids and electrolytes to replace body salts. If you develop diarrhea during your treatment with TRODELVY, your healthcare provider should check to see if it may be caused by an infection. Your healthcare provider may decrease your dose or stop TRODELVY if your diarrhea is severe and cannot be controlled with anti-diarrheal medicines.
- Call your healthcare provider right away the first time that you get diarrhea during treatment with TRODELVY; if you have black or bloody stools; if you have symptoms of dehydration, such as lightheadedness, dizziness, or faintness; if you are unable to take fluids by mouth due to nausea or vomiting; or if you are not able to get your diarrhea under control within 24 hours.
Do not receive TRODELVY if you have had a severe allergic reaction to TRODELVY. Ask your healthcare provider if you are not sure.
Allergic and infusion-related reactions which can be serious and life-threatening. Tell your healthcare provider or nurse right away if you get any of the following symptoms during your infusion of TRODELVY or within 24 hours after: swelling of your face, lips, tongue, or throat; hives; skin rash, itching, or flushing of your skin; fever; difficulty breathing or wheezing; lightheadedness, dizziness, feeling faint, or pass out; or chills or shaking chills (rigors).
Nausea and vomiting are common with TRODELVY and can sometimes be severe. Before each dose of TRODELVY, you will receive medicines to help prevent nausea and vomiting along with medicines to take home with instructions about how to take them. Call your healthcare provider right away if you have nausea or vomiting that is not controlled with the medicines prescribed for you. Your healthcare provider may decide to decrease your dose or stop TRODELVY if your nausea and vomiting is severe and cannot be controlled with anti-nausea medicines.
Before receiving TRODELVY, tell your healthcare provider about all of your medical conditions, including if you:
- have been told that you carry a gene for UGT1A1*28, which can increase your risk of getting side effects with TRODELVY, especially low white blood cell counts, with or without a fever, and low red blood cell counts.
- have liver problems.
- are pregnant or plan to become pregnant. TRODELVY can harm your unborn baby. Your healthcare provider should check to see if you are pregnant before you start receiving TRODELVY. TRODELVY may cause fertility problems in females, which could affect your ability to have a baby. Talk to your healthcare provider if fertility is a concern for you.
- Females who can become pregnant should use effective birth control during treatment and for 6 months after your last dose of TRODELVY. Talk to your healthcare provider about birth control choices that may be right for you during this time. Tell your healthcare provider right away if you become pregnant during treatment with TRODELVY.
- Males with a female partner who can become pregnant should use effective birth control during treatment and for 3 months after your last dose of TRODELVY.
- are breastfeeding or plan to breastfeed. It is not known if TRODELVY passes into your breastmilk and can harm your baby. Do not breastfeed during treatment and for 1 month after your last dose of TRODELVY.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain medicines may affect the way TRODELVY works.
The most common side effects of TRODELVY include decreased white blood cell (leukocyte and lymphocyte) and red blood cell counts, feeling tired or weak, hair loss, constipation, increased sugar levels in the blood, decreased protein levels (albumin) in the blood, decreased appetite, changes in kidney function test, increased levels of enzyme called alkaline phosphatase in the blood (test for liver or bone problems), and decreased levels of magnesium, potassium, and sodium in the blood.
These are not all of the possible side effects of TRODELVY. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
WHAT IS TRODELVY?
TRODELVY® (sacituzumab govitecan-hziy) is a prescription medicine used to treat adults with:
- triple-negative breast cancer (negative for estrogen and progesterone hormone receptors and HER2) that has spread to other parts of the body (metastatic) or cannot be removed by surgery, and who have received two or more prior treatments, including at least one treatment for metastatic disease.
- hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative breast cancer that has spread to other parts of the body or cannot be removed by surgery, and who previously received endocrine therapy and at least two additional treatments for metastatic disease.
- bladder cancer and cancers of the urinary tract that have spread or cannot be removed by surgery, and who have received a platinum-containing chemotherapy medicine and also received an immunotherapy medicine. This indication is approved based on medical studies that measured how many patients responded and how long they responded. Continued approval may depend on benefit demonstrated in additional medical studies.
It is not known if TRODELVY is safe and effective in people with moderate or severe liver problems or in children.
Please see Important Facts, including Important Warning on TRODELVY.com.
<END OF VOICEOVER AND TEXT ON-SCREEN>
Crystal:
I just learned this for the first time, so I recommend you follow up with your doctor to see if TRODELVY is right for you.
<TEXT ON-SCREEN>
TRODELVY®
sacituzumab govitecan-hziy
180 mg for injection
GILEAD
This information does not constitute the provision of medical advice and should not substitute for clinical decision making.
TRODELVY, the TRODELVY logo, GILEAD, and the GILEAD logo are trademarks of Gilead Sciences, Inc., or its related companies. All other marks are the property of their respective owners.
© 2023 Gilead Sciences, Inc. All rights reserved. US-TROC-0123 02/23
<END OF TEXT ON-SCREEN>
<TEXTO EN PANTALLA>
CÓMO TRODELVY ESTÁ DISEÑADO PARA ACTUAR
TRODELVY®
Sacituzumab govitecan-hziy
180 mg inyectable
¿QUÉ ES TRODELVY?
TRODELVY® (sacituzumab govitecan-hziy) es un medicamento de venta con receta que se usa para tratar a adultos con lo siguiente:
- Cáncer de mama triple negativo (negativo para receptores hormonales de estrógenos y progesterona y HER2) que se ha extendido a otras partes del cuerpo (metastásico) o no se puede extirpar mediante cirugía, y que han recibido dos o más tratamientos previos, incluido al menos un tratamiento para la enfermedad metastásica.
- Cáncer de mama con receptor hormonal (RH) positivo y receptor 2 del factor de crecimiento epidérmico humano (HER2) negativo que se ha extendido a otras partes del cuerpo o que no se puede extirpar mediante cirugía, y que ha recibido previamente terapia endocrina y al menos dos tratamientos adicionales para la enfermedad metastásica.
- Cáncer de vejiga y cánceres de las vías urinarias que se han extendido o no se pueden extirpar mediante cirugía, y que han recibido un medicamento en quimioterapia con platino y también un medicamento de inmunoterapia. Se aprueba esta indicación en función de estudios médicos que midieron cuántos pacientes respondieron y cuánto tiempo respondieron. La continuidad de la aprobación puede depender del beneficio demostrado en estudios médicos adicionales.
Se desconoce si TRODELVY es seguro y eficaz en personas con problemas hepáticos moderados o graves o en niños.
INFORMACIÓN IMPORTANTE DE SEGURIDAD
TRODELVY puede causar efectos secundarios graves, como un recuento bajo de glóbulos blancos y diarrea:
- El recuento bajo de glóbulos blancos (neutropenia) es frecuente y, a veces, puede ser grave y provocar infecciones que pueden ser potencialmente mortales o causar la muerte. Su proveedor de atención médica debe comprobar sus recuentos de células sanguíneas durante el tratamiento. Si su recuento de glóbulos blancos es demasiado bajo, es posible que su proveedor de atención médica necesite reducir su dosis, administrarle un medicamento para ayudar a prevenir un recuento bajo de células sanguíneas con dosis futuras de TRODELVY o, en algunos casos, interrumpir TRODELVY. Es posible que su proveedor de atención médica necesite administrarle antibióticos si presenta fiebre mientras su recuento de glóbulos blancos es bajo. Llame inmediatamente a su proveedor de atención médica si presenta alguno de los siguientes signos de infección: fiebre, escalofríos, tos, falta de aliento, o ardor o dolor al orinar.
- Diarrea grave. La diarrea es frecuente y puede ser grave. La diarrea intensa puede provocar la pérdida de demasiado líquido corporal (deshidratación) y problemas renales. Su proveedor de atención médica debe monitorear si tiene diarrea y administrarle medicamentos según sea necesario para ayudarle a controlarla. Si pierde demasiado líquido corporal, es posible que su proveedor de atención médica deba administrarle líquidos y electrolitos para sustituir las sales corporales. Si tiene diarrea durante su tratamiento con TRODELVY, su proveedor de atención médica debe comprobar si una infección puede ser la causa. Su proveedor de atención médica puede disminuir su dosis o interrumpir TRODELVY si su diarrea es grave y no puede controlarse con medicamentos antidiarreicos.
- Llame a su proveedor de atención médica de inmediato la primera vez que tenga diarrea durante el tratamiento con TRODELVY; si tiene heces negras o con sangre; si tiene síntomas de deshidratación, como aturdimiento, mareos o desmayos; si no puede tomar líquidos por vía oral debido a náuseas o vómitos; o si no puede controlar la diarrea en un plazo de 24 horas.
No reciba TRODELVY si ha tenido una reacción alérgica grave a TRODELVY. Si no está seguro, pregunte a su proveedor de atención médica.
Siga viendo este video hasta el final para obtener la Información importante de seguridad y consulte el enlace proporcionado para ver los datos importantes, incluida la Advertencia importante.
Los actores que aparecen en este video no son pacientes reales de TRODELVY. Están aquí para darle información y entretenerlo. Disfrute.
<FIN DEL TEXTO EN PANTALLA>
Crystal:
Soy un nerd de la información. Avistamiento de OVNIS, apicultura o los grandes éxitos de Barry Manilow, lo que sea, lo sé. Por eso, cuando me diagnosticaron cáncer metastásico, quise saber todo sobre las opciones de tratamiento.
<TEXTO EN PANTALLA>
TRODELVY®
sacituzumab govitecan-hziy
180 mg inyectable
<FIN DEL TEXTO EN PANTALLA>
Ahora, estoy viendo algunas experiencias de primera mano con TRODELVY sacituzumab govitecan-hziy.
<TEXTO EN PANTALLA>
sacituzumab govitecan-hziy
<FIN DEL TEXTO EN PANTALLA>
¿Eso es una palabra? ¿Alguien lo ha probado?
<TEXTO EN PANTALLA>
CRYSTAL
Tiene curiosidad por TRODELVY
Se autoproclama nerd de la información
<FIN DEL TEXTO EN PANTALLA>
<TEXTO EN PANTALLA>
¿TRODELVY? Todavía no lo he probado.
Tendré que hablar con mi médico sobre eso.
Mi médico me mencionó TRODELVY, pero no lo he investigado.
Hace 3 meses que tomo TRODELVY. ¿Qué tienes en mente?
He oído hablar de TRODELVY, pero también necesito obtener más información. Dime lo que aprendas.
<FIN DEL TEXTO EN PANTALLA>
Amanda:
Hace 3 meses que tomo TRODELVY. ¿Qué tienes en mente?
<TEXTO EN PANTALLA>
AMANDA
Paciente y defensora de TRODELVY
<FIN DEL TEXTO EN PANTALLA>
Crystal:
Tengo como 100 preguntas, pero ¿cómo funciona exactamente?
Amanda:
Mi médico me dijo que administra medicamentos contra el cáncer a las células. Pero cómo lo hace… Soy mala para explicar. Espera un momento.
<TEXTO EN PANTALLA>
Mi médico me dijo que administra medicamentos contra el cáncer a las células.
<FIN DEL TEXTO EN PANTALLA>
<TEXTO EN PANTALLA>
DRA. ESPOSITO
Oncóloga
(No sabe que está en silencio)
<FIN DEL TEXTO EN PANTALLA>
Amanda:
Está silenciada.
Dra. Esposito:
Perdón. Bueno, eh. Soy oncóloga. Me alegro de volver a verte. ¿Más gente quiere saber sobre TRODELVY? ¡Ya van 4 esta semana!
Crystal:
Esperen, ¿ustedes planearon esto?
Dra. Esposito:
Miremos las células cancerosas. Son inteligentes, difíciles de destruir y pueden volverse resistentes o no responder en absoluto al tratamiento.
TRODELVY adopta un enfoque diferente para las células cancerosas.
Crystal:
Bueno. Hasta ahora entiendo.
Dra. Esposito:
TRODELVY se conoce como un conjugado de anticuerpo y fármaco o CAF.
<TEXTO EN PANTALLA>
Conjugado de anticuerpo y fármaco
CAF
<FIN DEL TEXTO EN PANTALLA>
Amanda:
Eh, ¿anti qué?
Dra. Esposito:
TRODELVY es como un barco de carga. El barco de TRODELVY es un anticuerpo y lleva el medicamento contra el cáncer.
Jim:
Uh, no es por desanimarte, pero sé algo sobre barcos. Necesitan algún tipo de navegación para ver adónde van. Una vez me perdí en el mar y tuve que navegar usando el olfato. Solo teníamos esponjas de mar para comer y…
<TEXTO EN PANTALLA>
JIM
Paciente de TRODELVY
Perdido en el mar
<FIN DEL TEXTO EN PANTALLA>
Dra. Esposito:
No conozco los beneficios nutricionales de la esponja de mar. El SS TRODELVY es un anticuerpo diseñado para buscar una proteína llamada Trop-2. Comparadas con las células sanas, algunas células cancerosas pueden tener más Trop-2.
Jim:
Trop-2... ¿Tengo que ver la primera Trop para entender la secuela?
Amanda:
Buen chiste. ¿Estas Trop-2 le permiten a TRODELVY atacar las células?
Dra. Esposito:
La Trop-2 es como una señal que le indica a TRODELVY que entre en la zona de atraque de esa célula específica.
Jim:
¿Qué tipo de carga lleva este barco TRODELVY?
Dra. Esposito:
El que combate el cáncer. Cada barco TRODELVY puede llevar entre 7 y 8 moléculas de medicamento contra el cáncer.
Crystal:
¡Santo barco!
Dra. Esposito:
Cuando TRODELVY atraca en la célula, libera el medicamento contra el cáncer y destruye la célula.
Crystal:
¡Guau! ¿Así que TRODELVY actúa sobre las células con Trop-2? Parece un tratamiento que me gustaría probar. Lo hablaré con mi médico.
Dra. Esposito:
La información de los estudios de laboratorio sugiere que así es como funciona TRODELVY. Se desconoce el beneficio clínico de estas observaciones.
<TEXTO EN PANTALLA>
La información de los estudios de laboratorio sugiere que así es como funciona TRODELVY. Se desconoce el beneficio clínico de estas observaciones.
<FIN DEL TEXTO EN PANTALLA>
Crystal:
Gracias por explicarme cómo está diseñado para funcionar TRODELVY. ¿Hay alguna Información importante de seguridad que deba conocer?
[Narración con texto en pantalla]:
Me alegra que preguntes. Esto es lo que debes saber.
INFORMACIÓN IMPORTANTE DE SEGURIDAD
TRODELVY puede causar efectos secundarios graves, como un recuento bajo de glóbulos blancos y diarrea:
- El recuento bajo de glóbulos blancos (neutropenia) es frecuente y, a veces, puede ser grave y provocar infecciones que pueden ser potencialmente mortales o causar la muerte. Su proveedor de atención médica debe comprobar sus recuentos de células sanguíneas durante el tratamiento. Si su recuento de glóbulos blancos es demasiado bajo, es posible que su proveedor de atención médica necesite reducir su dosis, administrarle un medicamento para ayudar a prevenir un recuento bajo de células sanguíneas con dosis futuras de TRODELVY o, en algunos casos, interrumpir TRODELVY. Es posible que su proveedor de atención médica necesite administrarle antibióticos si presenta fiebre mientras su recuento de glóbulos blancos es bajo. Llame inmediatamente a su proveedor de atención médica si presenta alguno de los siguientes signos de infección: fiebre, escalofríos, tos, falta de aliento, o ardor o dolor al orinar.
- Diarrea grave. La diarrea es frecuente y puede ser grave. La diarrea intensa puede provocar la pérdida de demasiado líquido corporal (deshidratación) y problemas renales. Su proveedor de atención médica debe monitorear si tiene diarrea y administrarle medicamentos según sea necesario para ayudarle a controlarla. Si pierde demasiado líquido corporal, es posible que su proveedor de atención médica deba administrarle líquidos y electrolitos para sustituir las sales corporales. Si tiene diarrea durante su tratamiento con TRODELVY, su proveedor de atención médica debe comprobar si una infección puede ser la causa. Su proveedor de atención médica puede disminuir su dosis o interrumpir TRODELVY si su diarrea es grave y no puede controlarse con medicamentos antidiarreicos.
- Llame a su proveedor de atención médica de inmediato la primera vez que tenga diarrea durante el tratamiento con TRODELVY; si tiene heces negras o con sangre; si tiene síntomas de deshidratación, como aturdimiento, mareos o desmayos; si no puede tomar líquidos por vía oral debido a náuseas o vómitos; o si no puede controlar la diarrea en un plazo de 24 horas.
No reciba TRODELVY si ha tenido una reacción alérgica grave a TRODELVY. Si no está seguro, pregunte a su proveedor de atención médica.
Las reacciones alérgicas y relacionadas con la infusión pueden ser graves y potencialmente mortales. Informe inmediatamente a su proveedor de atención médica o al personal de enfermería si experimenta alguno de los siguientes síntomas durante la infusión de TRODELVY o dentro de las 24 horas siguientes: hinchazón de la cara, de los labios, de la lengua o de la garganta; urticaria; erupción cutánea, picazón o rubor en la piel; fiebre; dificultad para respirar o sibilancias; aturdimiento, mareos, sensación de desmayo o desmayo; escalofríos o escalofríos violentos (infecciosos intensos y de comienzo brusco).
Las náuseas y los vómitos son frecuentes con TRODELVY y a veces pueden ser graves. Antes de cada dosis de TRODELVY, recibirá medicamentos para ayudar a prevenir las náuseas y los vómitos, junto con medicamentos para llevarse a casa e instrucciones sobre cómo tomarlos. Llame a su proveedor de atención médica inmediatamente si tiene náuseas o vómitos que no puede controlar con los medicamentos que se le han recetado. Su proveedor de atención médica puede disminuir su dosis o interrumpir TRODELVY si sus náuseas y vómitos son graves y no pueden controlarse con medicamentos antináuseas.
Antes de recibir TRODELVY, informe a su proveedor de atención médica acerca de todas sus afecciones médicas, incluso en los siguientes casos:
- Se le ha informado que usted es portador de un gen para UGT1A1*28, que puede aumentar su riesgo de sufrir efectos secundarios con TRODELVY, especialmente recuentos bajos de glóbulos blancos, con o sin fiebre, y recuentos bajos de glóbulos rojos.
- Tiene problemas hepáticos.
- Está embarazada o planifica quedar embarazada. TRODELVY puede dañar al feto. Su proveedor de atención médica debe comprobar si está embarazada antes de empezar a recibir TRODELVY. TRODELVY puede causar problemas de fertilidad en las mujeres, lo que podría afectar a su capacidad para tener un bebé. Hable con su proveedor de atención médica si le preocupa la fertilidad.
- Las mujeres que pueden quedar embarazadas deben usar un método anticonceptivo eficaz durante el tratamiento y hasta los 6 meses posteriores a la última dosis de TRODELVY. Hable con su proveedor de atención médica acerca de los métodos anticonceptivos que pueden ser adecuados para usted durante este tiempo. Si queda embarazada durante el tratamiento con TRODELVY, informe de inmediato a su proveedor de atención médica.
- Los hombres con una pareja femenina que puedan quedar embarazadas deben utilizar un anticonceptivo eficaz durante el tratamiento y hasta 3 meses después de su última dosis de TRODELVY.
- Está amamantando o planifica amamantar. Se desconoce si TRODELVY pasa a la leche materna y puede dañar a su bebé. No amamante durante el tratamiento ni hasta 1 mes después de su última dosis de TRODELVY.
Informe a su proveedor de atención médica sobre todos los medicamentos que toma, incluidos los que se venden con receta y los de venta libre, vitaminas y suplementos a base de hierbas. Ciertos medicamentos pueden afectar el funcionamiento de TRODELVY.
Entre los efectos secundarios más frecuentes de TRODELVY se incluyen disminución del recuento de glóbulos blancos (leucocitos y linfocitos) y de glóbulos rojos, sensación de cansancio o debilidad, caída del cabello, estreñimiento, aumento de los niveles de azúcar en sangre, disminución de los niveles de proteínas (albúmina) en sangre, disminución del apetito, cambios en la prueba de función renal, aumento de los niveles de una enzima llamada fosfatasa alcalina en sangre (prueba de problemas hepáticos u óseos), disminución de los niveles de magnesio, potasio y sodio en la sangre.
Estos no son todos los efectos secundarios posibles de TRODELVY. Llame a su médico para que lo asesore acerca de los efectos secundarios. Usted puede informar los efectos secundarios a la FDA llamando al 1-800-FDA-1088.
¿QUÉ ES TRODELVY?
TRODELVY® (sacituzumab govitecan-hziy) es un medicamento de venta con receta que se usa para tratar a adultos con lo siguiente:
- Cáncer de mama triple negativo (negativo para receptores hormonales de estrógenos y progesterona y HER2) que se ha extendido a otras partes del cuerpo (metastásico) o no se puede extirpar mediante cirugía, y que han recibido dos o más tratamientos previos, incluido al menos un tratamiento para la enfermedad metastásica.
- Cáncer de mama con receptor hormonal (RH) positivo y receptor 2 del factor de crecimiento epidérmico humano (HER2) negativo que se ha extendido a otras partes del cuerpo o que no se puede extirpar mediante cirugía, y que ha recibido previamente terapia endocrina y al menos dos tratamientos adicionales para la enfermedad metastásica.
- Cáncer de vejiga y cánceres de las vías urinarias que se han extendido o no se pueden extirpar mediante cirugía, y que han recibido un medicamento en quimioterapia con platino y también un medicamento de inmunoterapia. Se aprueba esta indicación en función de estudios médicos que midieron cuántos pacientes respondieron y cuánto tiempo respondieron. La continuidad de la aprobación puede depender del beneficio demostrado en estudios médicos adicionales.
Se desconoce si TRODELVY es seguro y eficaz en personas con problemas hepáticos moderados o graves o en niños.
Consulte los Datos importantes sobre TRODELVY, incluida la Advertencia importante en TRODELVY.com.
<FIN DE LA VOZ EN OFF Y DEL TEXTO EN PANTALLA>
Crystal:
Acabo de enterarme de esto, así que te recomiendo que consultes a tu médico para ver si TRODELVY es adecuado para ti.
<TEXTO EN PANTALLA>
TRODELVY®
sacituzumab govitecan-hziy
180 mg inyectable
GILEAD
Esta información no constituye asesoramiento médico y no debe sustituir a la toma de decisiones clínicas.
TRODELVY, el logotipo de TRODELVY, GILEAD y el logotipo de GILEAD son marcas comerciales de Gilead Sciences, Inc. o de sus compañías relacionadas. Las otras marcas son propiedad de sus respectivos dueños.
© 2023 Gilead Sciences, Inc. Todos los derechos reservados. US-TROC-0123 02/23
<FIN DEL TEXTO EN PANTALLA>
TRODELVY® (sacituzumab govitecan-hziy) is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract that have spread (metastatic) or cannot be removed by surgery, and who have received a platinum-containing chemotherapy medicine and also received an immunotherapy medicine.
This indication is approved based on medical studies that measured how many patients responded and how long they responded. Continued approval may depend on benefit demonstrated in additional medical studies.
It is not known if TRODELVY is safe and effective in people with moderate or severe liver problems or in children.
Important Safety Information
TRODELVY can cause serious side effects, including low white blood cell count and diarrhea:
Tap for Important Safety Information. TRODELVY can cause serious side effects, including low white blood cell count and diarrhea:
- Low white blood cell count (neutropenia) which is common and can sometimes be severe and lead to infections that can be life-threatening or cause death. Your healthcare provider should check your blood cell counts during treatment. If your white blood cell count is too low, your healthcare provider may need to lower your dose, give you a medicine to help prevent low blood cell count with future doses of TRODELVY, or in some cases may stop TRODELVY. Your healthcare provider may need to give you antibiotic medicines if you develop fever while your white blood cell count is low. Call your healthcare provider right away if you develop any of the following signs of infection: fever, chills, cough, shortness of breath, or burning or pain when you urinate.
- Severe diarrhea. Diarrhea is common and can be severe. Severe diarrhea can lead to loss of too much body fluid (dehydration) and kidney problems. Your healthcare provider should monitor you for diarrhea and give you medicine as needed to help control it. If you lose too much body fluid, your healthcare provider may need to give you fluids and electrolytes to replace body salts. If you develop diarrhea during your treatment with TRODELVY, your healthcare provider should check to see if it may be caused by an infection. Your healthcare provider may decrease your dose or stop TRODELVY if your diarrhea is severe and cannot be controlled with anti-diarrheal medicines.
- Call your healthcare provider right away the first time that you get diarrhea during treatment with TRODELVY; if you have black or bloody stools; if you have symptoms of dehydration, such as lightheadedness, dizziness, or faintness; if you are unable to take fluids by mouth due to nausea or vomiting; or if you are not able to get your diarrhea under control within 24 hours.
Do not receive TRODELVY if you have had a severe allergic reaction to TRODELVY. Ask your healthcare provider if you are not sure.
Allergic and infusion-related reactions which can be serious and life-threatening. Tell your healthcare provider or nurse right away if you get any of the following symptoms during your infusion of TRODELVY or within 24 hours after: swelling of your face, lips, tongue, or throat; hives; skin rash, itching, or flushing of your skin; fever; difficulty breathing or wheezing; lightheadedness, dizziness, feeling faint, or pass out; or chills or shaking chills (rigors).
Nausea and vomiting are common with TRODELVY and can sometimes be severe. Before each dose of TRODELVY, you will receive medicines to help prevent nausea and vomiting along with medicines to take home with instructions about how to take them. Call your healthcare provider right away if you have nausea or vomiting that is not controlled with the medicines prescribed for you. Your healthcare provider may decide to decrease your dose or stop TRODELVY if your nausea and vomiting is severe and cannot be controlled with anti-nausea medicines.
Before receiving TRODELVY, tell your healthcare provider about all of your medical conditions, including if you:
- have been told that you carry a gene for UGT1A1*28, which can increase your risk of getting side effects with TRODELVY, especially low white blood cell counts, with or without a fever, and low red blood cell counts.
- have liver problems.
- are pregnant or plan to become pregnant. TRODELVY can harm your unborn baby. Your healthcare provider should check to see if you are pregnant before you start receiving TRODELVY. TRODELVY may cause fertility problems in females, which could affect your ability to have a baby. Talk to your healthcare provider if fertility is a concern for you.
- Females who can become pregnant should use effective birth control during treatment and for 6 months after your last dose of TRODELVY. Talk to your healthcare provider about birth control choices that may be right for you during this time. Tell your healthcare provider right away if you become pregnant during treatment with TRODELVY.
- Males with a female partner who can become pregnant should use effective birth control during treatment and for 3 months after your last dose of TRODELVY.
- are breastfeeding or plan to breastfeed. It is not known if TRODELVY passes into your breastmilk and can harm your baby. Do not breastfeed during treatment and for 1 month after your last dose of TRODELVY.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain medicines may affect the way TRODELVY works.
The most common side effects of TRODELVY include decreased white blood cell (leukocyte and lymphocyte) and red blood cell counts, feeling tired or weak, hair loss, constipation, increased sugar levels in the blood, decreased protein levels (albumin) in the blood, decreased appetite, changes in kidney function test, increased levels of enzyme called alkaline phosphatase in the blood (test for liver or bone problems), and decreased levels of magnesium, potassium, and sodium in the blood.
These are not all of the possible side effects of TRODELVY. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please click to see Important Facts about TRODELVY, including Important Warning.


