TRODELVY® (sacituzumab govitecan-hziy) is a prescription medicine used to treat adults with hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative breast cancer that has spread to other parts of the body (metastatic) or cannot be removed by surgery, and who previously received endocrine therapy and at least two additional treatments for metastatic disease. It is not known if TRODELVY is safe and effective in people with moderate or severe liver problems or in children.
Clinical trial results in HR+/HER2- mBC
TRODELVY was tested in a large phase 3 clinical trial

Researchers tested TRODELVY in a phase 3 clinical trial of 543 adults with previously treated* HR+/HER2- mBC comparing TRODELVY to traditional chemotherapy to find out how long people:
*For information on previous treatments, click “Who were the participants?” below.
HER2- = human epidermal growth factor receptor 2-negative, HR+ = hormone receptor-positive, mBC = metastatic breast cancer

- The researchers selected the type of chemo they received
- Types of chemo included eribulin, capecitabine, gemcitabine, or vinorelbine

All patients had been previously diagnosed with HR+/HER2- mBC and had received
- endocrine therapy
- CDK4/6 inhibitor (a type of targeted therapy)
- taxane (a type of chemotherapy)
- at least 2 and no more than 4 prior chemotherapies for metastatic breast cancer
One of the chemotherapies could have been given as a neoadjuvant or adjuvant treatment if recurrence occurred within 12 months.
Endocrine therapy, CDK4/6 inhibitor, and taxane treatments were administered in a neoadjuvant, adjuvant, or metastatic setting.

TRODELVY is an intravenous infusion.
In the clinical trial, patients who were given TRODELVY received 10 mg/kg on days 1 and 8 of a 21-day treatment cycle.
Researchers determined when to stop TRODELVY treatment depending on how patients were responding to and tolerating treatment.
Interested in TRODELVY?
If you have received endocrine therapy and 2 or more treatments for your HR+/HER2- metastatic breast cancer, TRODELVY may be an option for you. Create a plan today for asking your doctor about TRODELVY.
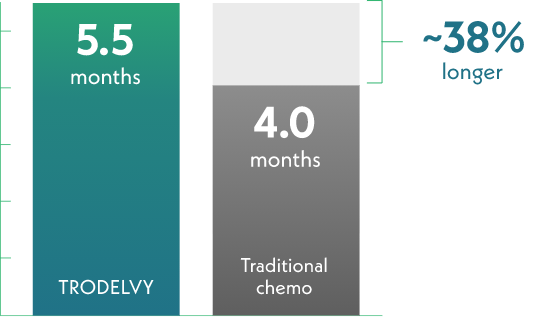
TRODELVY helped patients live longer than traditional chemotherapy
Half of those receiving TRODELVY lived without cancer progression about 38% longer
Traditional chemotherapy = 271 patients
This result is called median progression-free survival (PFS), which is how long a treatment stops the growth or spread of HR+/HER2- mBC in half of the people who take it.
TRODELVY
Half of those who received TRODELVY lived with no sign of cancer progression for 5.5 months.
Traditional chemo
Half of those who received traditional chemo (eribulin, vinorelbine, gemcitabine, or capecitabine) lived with no sign of cancer progression for 4 months.
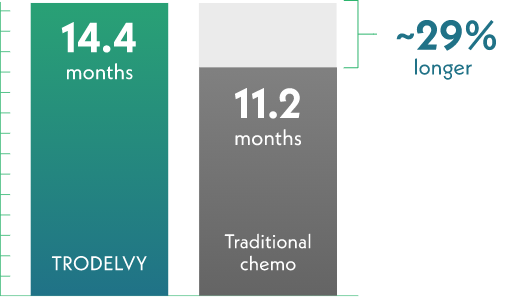
Half of those receiving TRODELVY lived about 29% longer overall
Traditional chemotherapy = 271 patients
This result is called median overall survival (OS), which is how long half of the people are alive after starting treatment.
TRODELVY
Half of those who received TRODELVY lived for over 1 year (14.4 months).
Traditional chemo
Half of those receiving traditional chemo lived for 11.2 months.
Researchers also studied how long it took before certain quality of life measures worsened

Health-related quality of life (HRQoL) is the way a person feels about their overall physical and mental health over a period of time.
In the clinical trial, researchers focused on HRQoL measures like overall health and quality of life, fatigue, and pain.

Researchers understand that there are significant health-related quality of life (HRQoL) measures for people living with HR+/HER2- mBC that are not commonly addressed.
That’s why they studied how long it took for certain measures of HRQoL to worsen for people receiving TRODELVY compared to traditional chemo.
Patients completed a 30-question survey* about how different measures of HRQoL, like overall health and quality of life, pain, and fatigue, affected them over the past week.
Patients completed this survey at the beginning of the trial and at least 1 other time during the trial.
Patients gave a numeric score to each question.
*The survey used was the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ).

Researchers wanted to find out how much time it took for certain quality of life measures to get worse for patients receiving TRODELVY compared to patients receiving traditional chemo.
Researchers calculated the median† amount of time it took for those scores to get worse by at least 10 points.
Finally, researchers determined if the difference between the median amount of time in the TRODELVY group and the traditional chemo group was statistically significant.‡
†The median is the middle of a set of numbers; in other words, about half of the people in the study experienced these results.
‡To be statistically significant, the difference between 2 groups must be greater than the difference that could happen by chance.
Certain measures of HRQoL took longer to worsen with TRODELVY than traditional chemo
Important to know: Consider these results carefully. The phase 3 trial was an open-label study. This means researchers knew which patients were receiving TRODELVY, and patients also knew which treatment they were receiving. The questionnaire used was not all-inclusive as it did not measure all treatment-related symptoms or how much the symptoms bothered patients. Also, the results shown here may be unrelated to treatment.
Median time until overall health and quality of life got worse
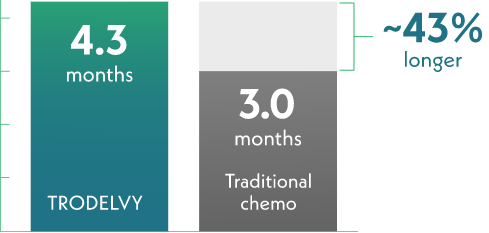
Traditional chemotherapy = 207 patients
Half of those receiving TRODELVY reported it took 1.3 months longer for overall health and quality of life to get worse.
Median time until fatigue got worse

Traditional chemotherapy = 205 patients
Half of those receiving TRODELVY reported it took almost 1 month longer for fatigue to get worse.
Median time until pain got worse
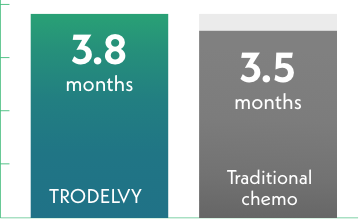
Traditional chemotherapy = 202 patients
Time until pain got worse was similar for both groups. This result was not a statistically significant difference.§
§To be statistically significant, the difference between 2 groups must be greater than the difference that could happen by chance.
TRODELVY® (sacituzumab govitecan-hziy) is a prescription medicine used to treat adults with hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative breast cancer that has spread to other parts of the body (metastatic) or cannot be removed by surgery, and who previously received endocrine therapy and at least two additional treatments for metastatic disease.
It is not known if TRODELVY is safe and effective in people with moderate or severe liver problems or in children.
Important Safety Information
TRODELVY can cause serious side effects, including low white blood cell count and diarrhea:
Tap for Important Safety Information. TRODELVY can cause serious side effects, including low white blood cell count and diarrhea:
- Low white blood cell count (neutropenia) is common and can sometimes be severe and lead to infections that can be life-threatening or cause death as early as the first cycle of treatment. Your healthcare provider should check your blood cell counts during treatment and may give a medicine to help prevent neutropenia starting in the first cycle of treatment if you have an increased risk for developing low white blood cell count with a fever (febrile neutropenia). If your white blood cell count is too low, your healthcare provider may need to delay treatment or lower your dose, give you a medicine to treat low blood cell count, or in some cases may permanently stop TRODELVY. Your healthcare provider may need to give you antibiotic medicines if you develop fever while your white blood cell count is low. Call your healthcare provider right away if you develop any of the following signs of infection: fever, chills, cough, shortness of breath, or burning or pain when you urinate.
- Severe diarrhea. Diarrhea is common and can be severe. Severe diarrhea can lead to loss of too much body fluid (dehydration) and kidney problems. Your healthcare provider should monitor you for diarrhea and give you medicine as needed to help control it. If you lose too much body fluid, your healthcare provider may need to give you fluids and electrolytes to replace body salts. If you develop diarrhea during your treatment with TRODELVY, your healthcare provider should check to see if it may be caused by an infection. Your healthcare provider may decrease your dose, delay treatment or permanently stop TRODELVY if your diarrhea is severe and cannot be controlled with anti-diarrheal medicines.
- Call your healthcare provider right away the first time that you get diarrhea during treatment with TRODELVY; if you have black or bloody stools; if you have symptoms of dehydration, such as lightheadedness, dizziness, or faintness; if you are unable to take fluids by mouth due to nausea or vomiting; or if you are not able to get your diarrhea under control within 24 hours.
Do not receive TRODELVY if you have had a severe allergic reaction to TRODELVY. Ask your healthcare provider if you are not sure.
Allergic and infusion-related reactions can be serious and life-threatening. Tell your healthcare provider or nurse right away if you get any of the following symptoms during your infusion of TRODELVY or within 24 hours after: swelling of your face, lips, tongue, or throat; hives; skin rash, itching, or flushing of your skin; fever; difficulty breathing or wheezing; lightheadedness, dizziness, feeling faint, or pass out; or chills or shaking chills (rigors).
Nausea and vomiting are common and can sometimes be severe. Before each dose of TRODELVY, you will receive medicines to help prevent nausea and vomiting along with medicines to take home with instructions about how to take them. Call your healthcare provider right away if you have nausea or vomiting that is not controlled with the medicines prescribed for you. Your healthcare provider may decide to decrease your dose, delay treatment, or permanently stop TRODELVY if your nausea and vomiting is severe and cannot be controlled with anti-nausea medicines.
Before receiving TRODELVY, tell your healthcare provider about all of your medical conditions, including if you:
- have been told that you carry a gene for UGT1A1*28, which can increase your risk of getting side effects with TRODELVY, especially low white blood cell counts, with or without a fever, and low red blood cell counts.
- have liver problems.
- are pregnant or plan to become pregnant. TRODELVY can harm your unborn baby. Your healthcare provider should check to see if you are pregnant before you start receiving TRODELVY. TRODELVY may cause fertility problems in females, which could affect your ability to have a baby. Talk to your healthcare provider if fertility is a concern for you.
- Females who can become pregnant should use effective birth control during treatment and for 6 months after your last dose of TRODELVY. Talk to your healthcare provider about birth control choices that may be right for you during this time. Tell your healthcare provider right away if you become pregnant during treatment with TRODELVY.
- Males with a female partner who can become pregnant should use effective birth control during treatment and for 3 months after your last dose of TRODELVY.
- are breastfeeding or plan to breastfeed. It is not known if TRODELVY passes into your breastmilk and can harm your baby. Do not breastfeed during treatment and for 1 month after your last dose of TRODELVY.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Certain medicines may affect the way TRODELVY works.
The most common side effects of TRODELVY include decreased white blood cell (leukocyte and lymphocyte) and red blood cell counts, feeling tired or weak, hair loss, constipation, increased sugar levels in the blood, decreased protein levels (albumin) in the blood, decreased appetite, changes in kidney function test, increased levels of enzyme called alkaline phosphatase in the blood (test for liver or bone problems), and decreased levels of magnesium, potassium, and sodium in the blood.
These are not all of the possible side effects of TRODELVY. Call your doctor for medical advice about side effects. You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Please click to see Important Facts about TRODELVY, including Important Warning.


